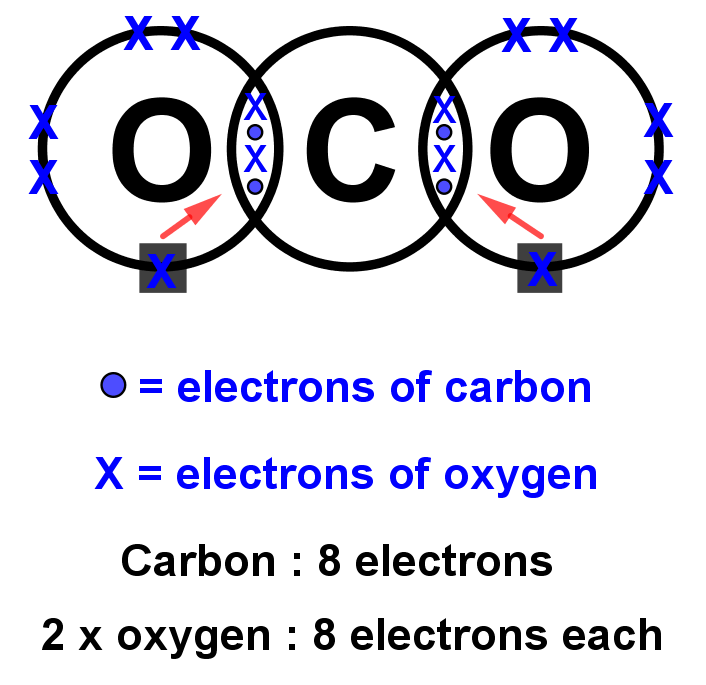
13+ Co2 Dot And Cross Diagram Robhosking Diagram
1. Introduction Electron configuration describes the distribution of electrons within an atom. The concept of electron configuration has been introduced since the discovery of Bohr atomic model. However, the electron configuration referred in this study was one that derived from the later atomic model - the quantum mechanics atomic model.

Carbon Electron Shell Diagram
The electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state . This handy chart compiles the electron configurations of the elements up through number 104.

Electronic Configuration for Carbon spdf Trick Chemistry Atomic
To determine the number of valence electrons for CO2, the Carbon dioxide molecule, we'll use the Periodic Table. Organizing the Periodic Table by Group, ski.
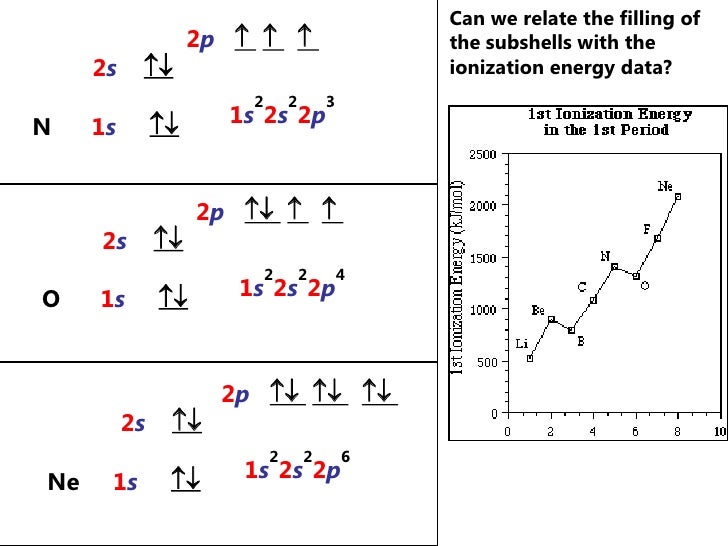
40 co3+ orbital diagram
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ).
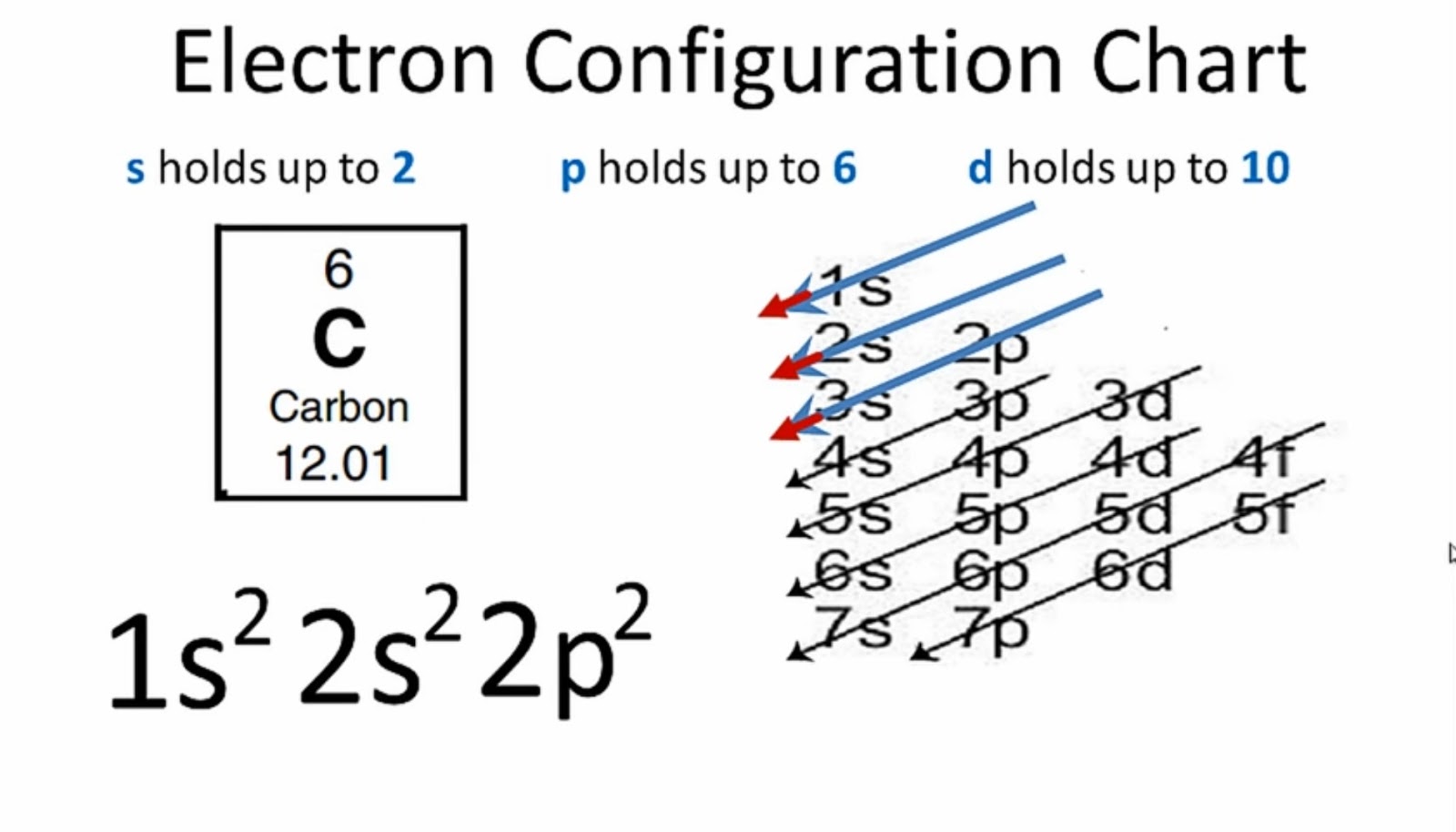
What Is the Carbon(C) Electron Configuration?
The n + l n + l rule tells you the order in which atomic orbitals are filled, and according to the rule the 4s 4 s orbital is occupied before the 3d 3 d orbital because it has lower energy. Thus, the electron configuration of Mn M n is [Ar]3d54s2 [ A r] 3 d 5 4 s 2 while that of Co C o is [Ar]3d74s2 [ A r] 3 d 7 4 s 2 .
Electron Configuration Of Carbon
In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.
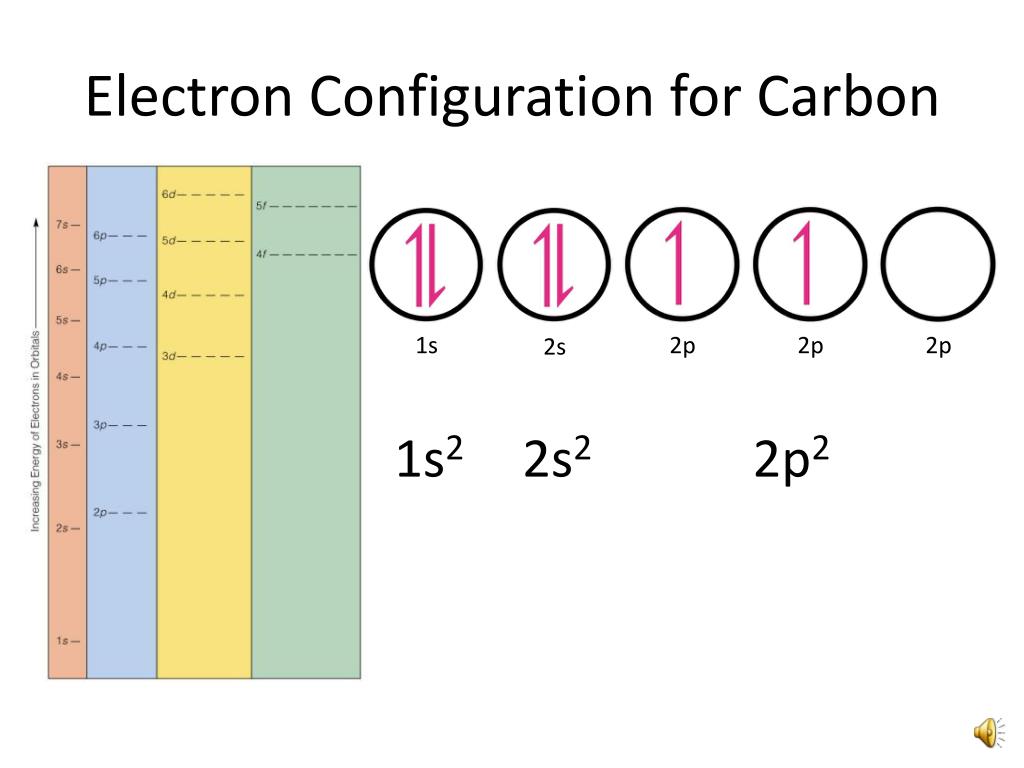
PPT Orbital Filling Electron Configurations PowerPoint Presentation
PROBLEM 3.1.13 3.1. 13. Thallium was used as a poison in the Agatha Christie mystery story "The Pale Horse.". Thallium has two possible cationic forms, +1 and +3. The +1 compounds are the more stable. Write the electron structure of the +1 cation of thallium. Answer.

Silver Electron Configuration
With ever-increasing atmospheric carbon dioxide concentrations and commitments to limit global temperatures to less than 1.5 °C above pre-industrial levels, the need for versatile, low-cost.
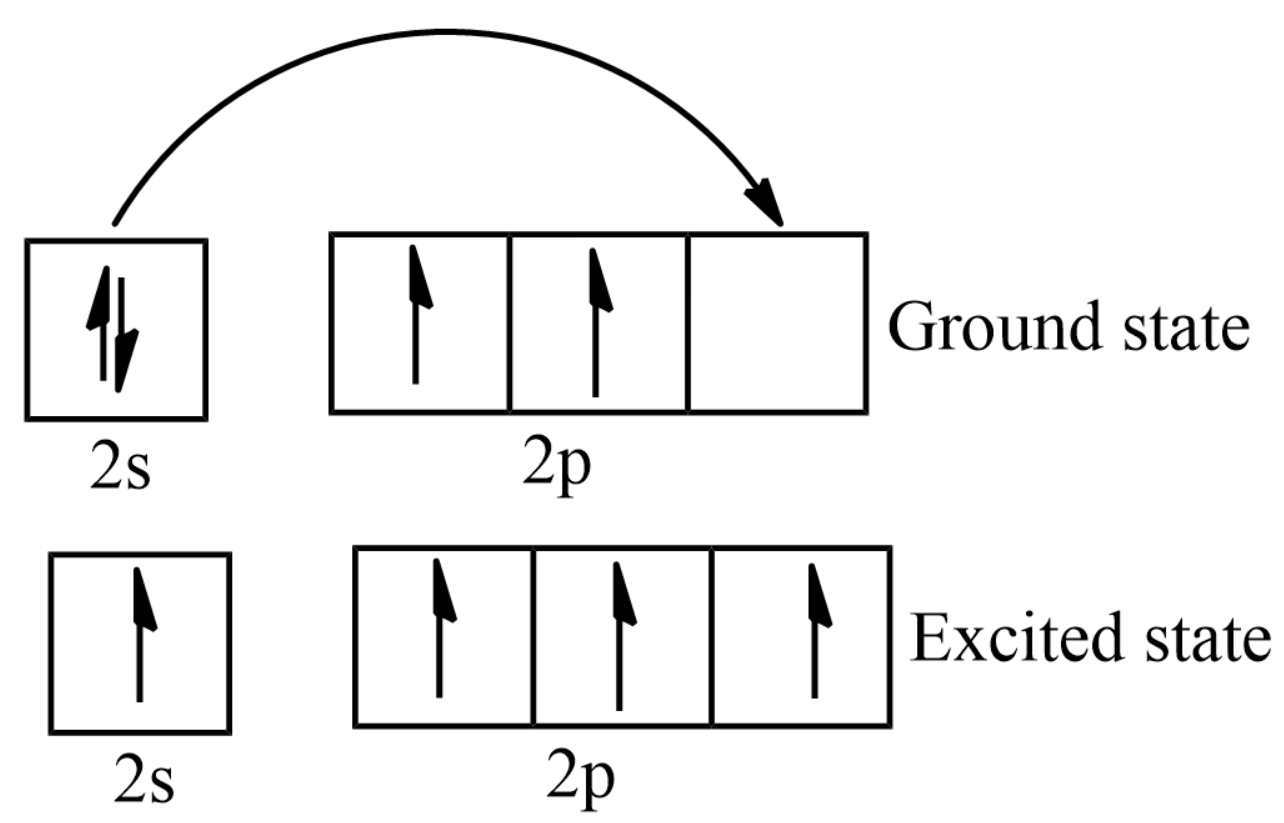
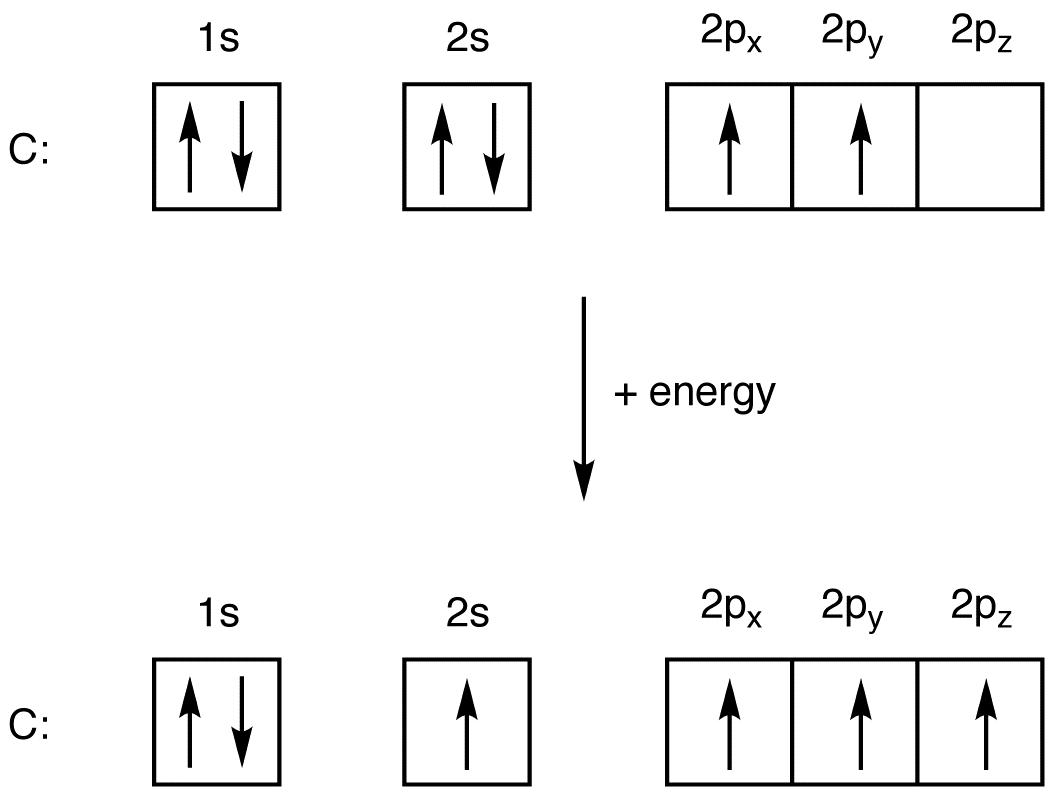
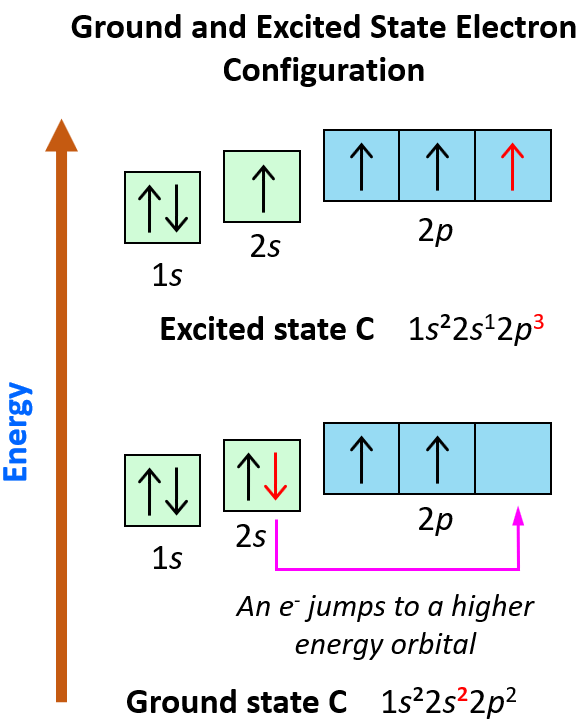
What is the excited state of carbon?
What is the electron configuration of cobalt? The total number of electrons in cobalt is twenty-seven. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in cobalt in specific rules in different orbits and orbitals is called the electron configuration of cobalt.

Carbon Electron Configuration
To write the configuration for the Cobalt ions, first we need to write the electron configuration for just Cobalt (Co). We first need to find the number of.
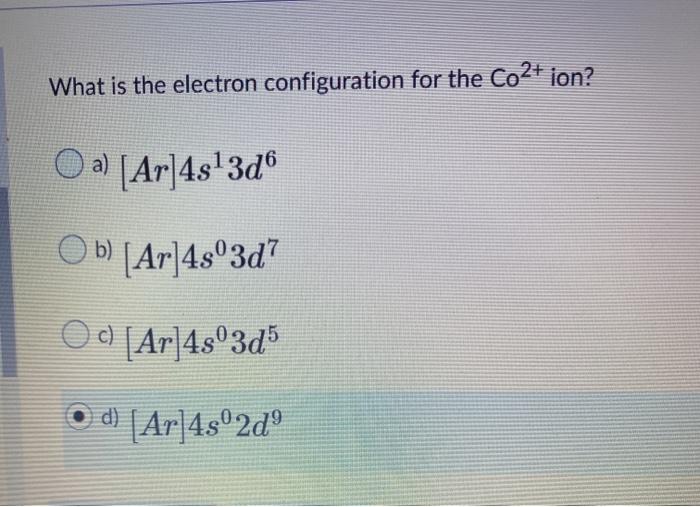
Solved What is the electron configuration for the Co2+ ion?
To write electron configuration of an element, locate its symbol in ADOMAH Periodic Table and cross out all elements that have higher atomic numbers. For example, if you need to write electron configuration of Erbium (68), cross out elements 69 through 120. Notice numbers 1 through 8 at the base of the table.
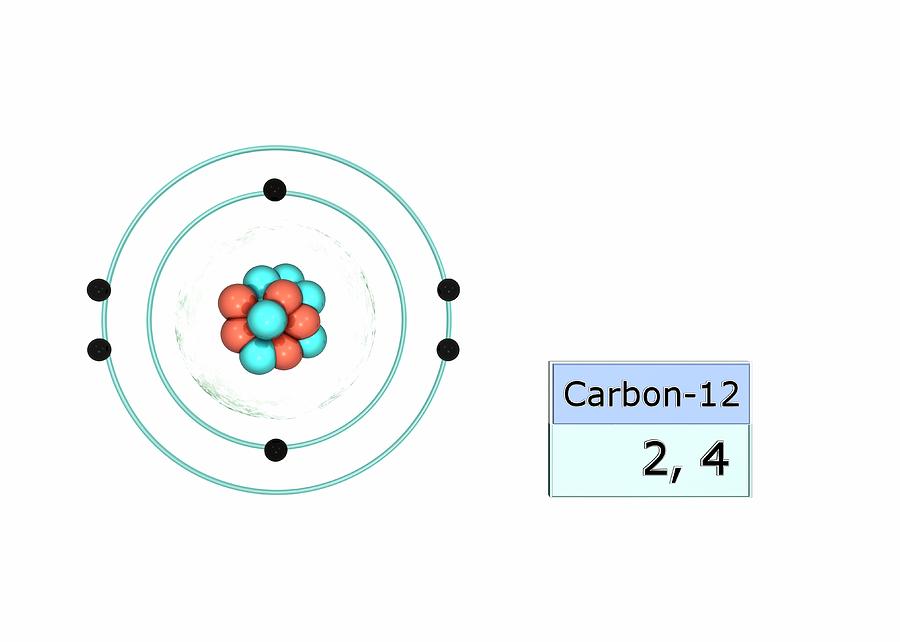
Carbon Electron Configuration Photograph by Photo
The electron configuration of Cobalt is [Ar]4s 2 3d 7. When observing Cobalt 3+, we know that Cobalt must lose three electrons. The first two to go are from the 4s orbital and Cobalt becomes:[Ar]4s 0 3d 7. Then, the next electron leaves the 3d orbital and the configuration becomes: [Ar]4s 0 3d 6. Thus, we can see that there are six electrons.
Electron Configurations Chemistry Steps
Hello Guys,Determining the electron configuration of any element is an easy and quick process, given that you know all the required information and general c.

Electron Configuration for Co, Co2+, and Co3+ (Cobalt and Cobalt Ions
The electron configuration of Co is 1s2 2s2 2p6 3s2 3p6 3d7. Co requires three more valence electrons in its d-orbital to attain full filled configuration. What is the complete electron configuration of Co 2+? The complete electron configuration of Co2+ is 1s2 2s 2 2p6 3s2 3p6 3d7.Co2+ have 7 valence electrons in the subshell.

Electron Geometry for CO2 (Carbon Dioxide) YouTube
Answer: The electron configurations of the elements are presented in Figure 2.2.3, which lists the orbitals in the order in which they are filled. In several cases, the ground state electron configurations are different from those predicted by Figure 2.2.1. Some of these anomalies occur as the 3 d orbitals are filled.

Orbital Diagram For Cobalt
Here, the electron configuration of cobalt ion(Co 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 7. This cobalt ion(Co 2+) has twenty-seven protons, thirty-two neutrons, and twenty-five electrons. Also, cobalt has one more ion. That is Co 3+. Co - 3e - → Co 3+ Here, the electron configuration of cobalt ion(Co 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6.